So, you’re sitting outside enjoying the high summer sun with the neighbor girl from next door you’ve been crushing on pretty hard. Your punchlines aren’t all that sharp, but she still humors you with a laugh because she was raised on good traditional values of hospitality. She motions for a glass of your “gotta try it” sweet tea you used as a reason to come over, of course, you oblige. Suddenly, as you pour your sly solution of sugar and shame, your inner geek rears its head out from behind a pair of Ray-Bans: why did that ice just float to the top like that? You know you placed it at the bottom of the cup before you poured the tea, so why is it now floating carelessly at the top of her glass? Worry not young man, I come bearing science.
To put it simply, what’s going on here is that the ice is less dense than the solution it is suspended in. Recalling our grade school science class, density is of course an object’s mass divided by its volume. Of course, I wouldn’t just leave it at that. No, there’s much more going on here than just a simple “just because” answer. We’re about to go a little more in depth into our icy inference.
At first though, one would say, well ice is just frozen water, why is its density different? A gram of water, should make a gram of ice right? Yes, but that would ignore both what is happening on a chemical level, and the other variable in calculating density: volume. This is where it gets interesting. As water molecules cool down they lose energy. As this happens something called hydrogen bonding begins to take place. What is happening is that the molecules will begin to form a very ordered crystalline structure. These new hydrogen bonds space the water molecules in a hexagonal arrangement and farther apart than they were in their liquid state, thus creating ice.
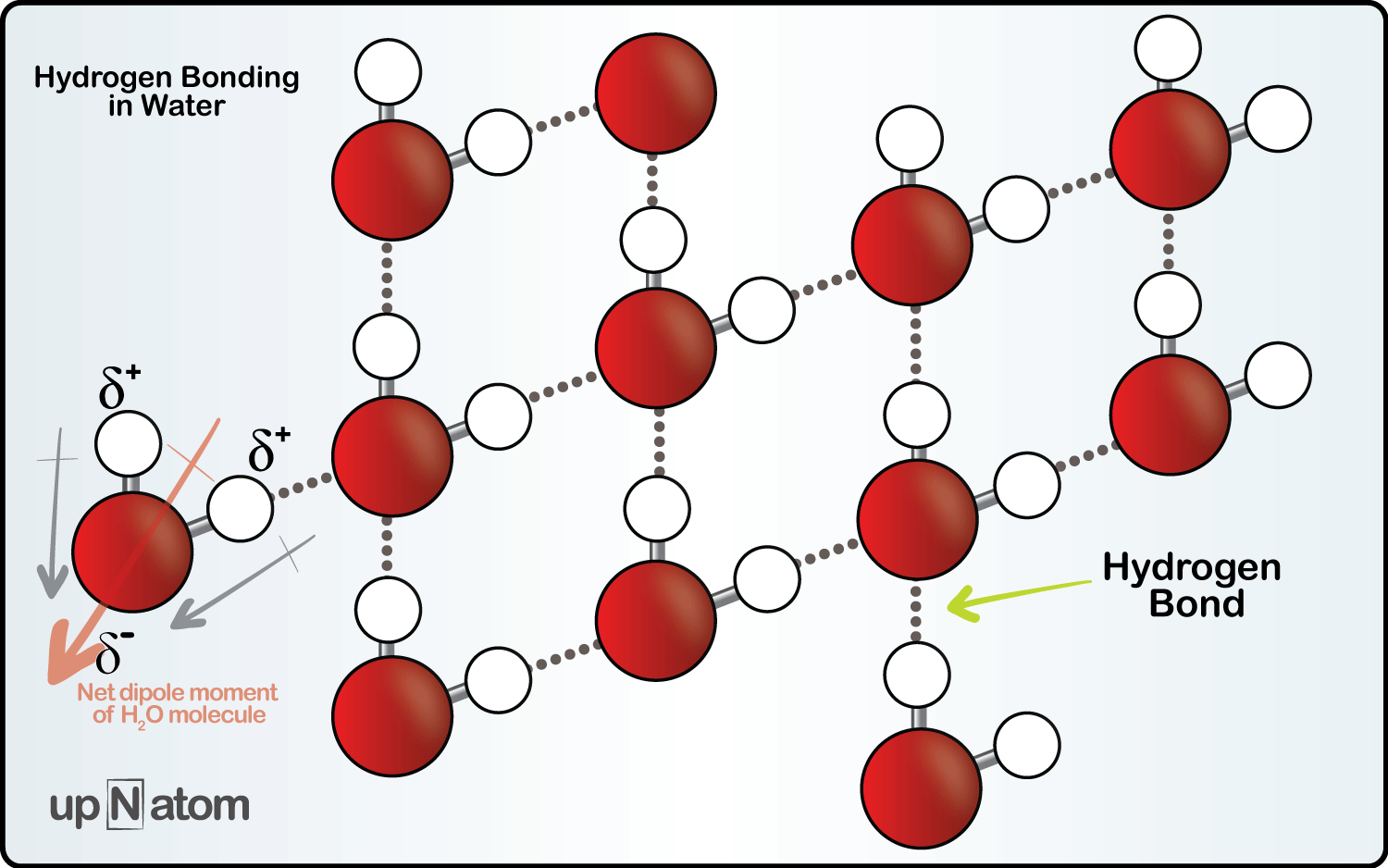
With that now in mind, let’s analyze the situation. Something that weighs the same as it did before, now occupies a much larger volume. Basic math will tell us if we take a number and divide it by a progressively larger number, naturally, the number will become progressively smaller. In other words, as the volume of the water increased when it became ice, the denominator of our density equation (ρ=m/v) becomes larger and our density value (ρ) becomes smaller. Most materials decrease in volume as they go into the solid state, Water on the other hand, is one of the few materials that increases in volume as it goes into a solid state. Wow, and here you thought the ice was just coming up for air.


